
3Rs working party

The EMA (European Medicines Agency) announced at the workshop the creation of a "3Rs working party" whose role will be to provide recommendations and help with the implementation of 3Rs measures. This should accelerate the transition to NAMs (New Approach Methodologies) in the pharmaceutical industry and the research world.
The EDQM (European Directorate for the Quality of Medicines & HealthCare) and the EPAA (European Partnership for Alternative Approaches to Animal Testing) organized a congress entitled "Phasing out the rabbit pyrogen test" from February 14 to 16 in Brussels, to explain and facilitate the replacement of the rabbit pyrogen test by in vitro alternatives, to identify the efforts that still need to be made in this respect, to encourage communication between the different actors of the sector (authorities, pharmaceutical industries, in vitro test suppliers, etc.) and this, on an international scale. In addition to the conferences, a morning of practical discussions around the implementation of in vitro tests was scheduled, and many representatives from outside the EU were present.
Pyrogens and their mode of action
Injectable products and medical devices intended to be implanted in the body (vaccines, antibiotics, blood and by-products, etc.) can potentially be contaminated during the manufacturing process, and are therefore subject to strict quality control to ensure patient safety and guarantee a similar and optimal result from one batch to the next. Potential contaminants - known as "pyrogens" - can come from a variety of sources (bacteria, viruses, yeast, molds), and trigger a fever reaction in the host through activation of the innate immune system, particularly monocytes. Pyrogens are generally classified into two groups: endotoxins (LPS from Gram-negative bacteria) and non-endotoxin pyrogens (NEPs).
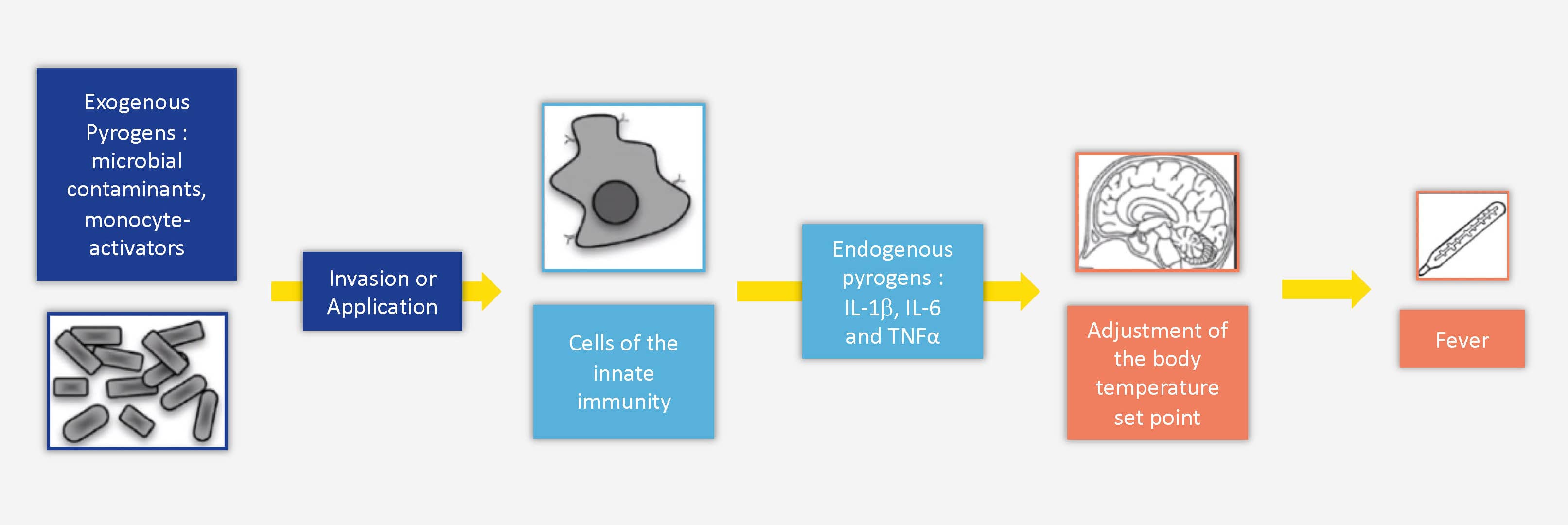
The rabbit pyrogen test
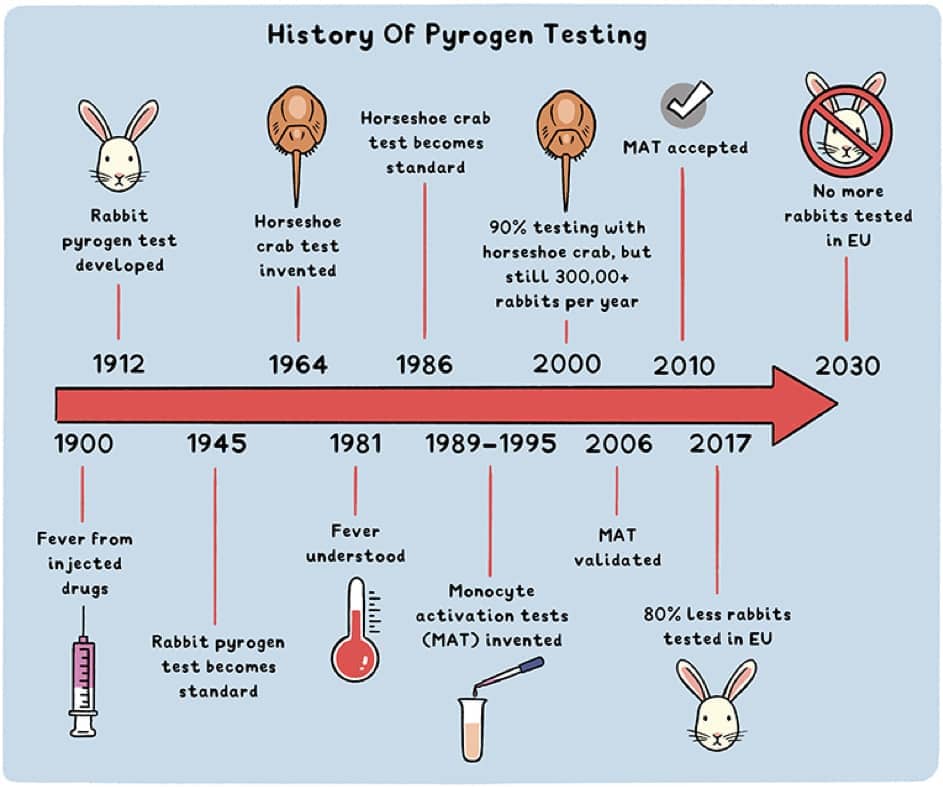
The rabbit pyrogen test, or RPT, developed in 1912 and present in the European Pharmacopoeia since 1986, consists of verifying the safety of products by testing their ability to induce fever in rabbits. But recently, this approach has been questioned for many reasons: the variability of the results obtained, the number of animals used and the impact on their well-being, the quantity of bacterial endotoxins necessary to cause a pyrogenic reaction, etc. Immunologists also warn about the reliability of these tests and the limitations of using the rabbit model, whose immune system is very different from that of humans (ref). It is high time to focus on the benefits of the different alternatives to the rabbit pyrogen test.
What are the alternatives?
- Endotoxin detection using Limulus (horseshoe crab) amebocyte lysate (LAL): The first alternative test to RPT, based on the coagulation of horseshoe crab blood in the presence of bacterial endotoxins was developed in 1964 and widely adopted since 1987 by regulatory authorities in the European Union and the rest of the world. However, it is a relative replacement, which is not neutral in terms of use and animal welfare. Indeed, these tests still require more than 500,000 horseshoe crabs per year. After blood sampling, they are then consumed - in Japan for example - or released, often too weakened to survive or reproduce.
- Since 2020, the BET (Bacterial Endotoxin Test), an in vitro test based on the principle of LAL, but using a recombinant synthetic factor (factor C "rFC") in a simplified biochemical reaction, allows for the detection of endotoxins in a simpler, more reliable and reproducible way than LAL.
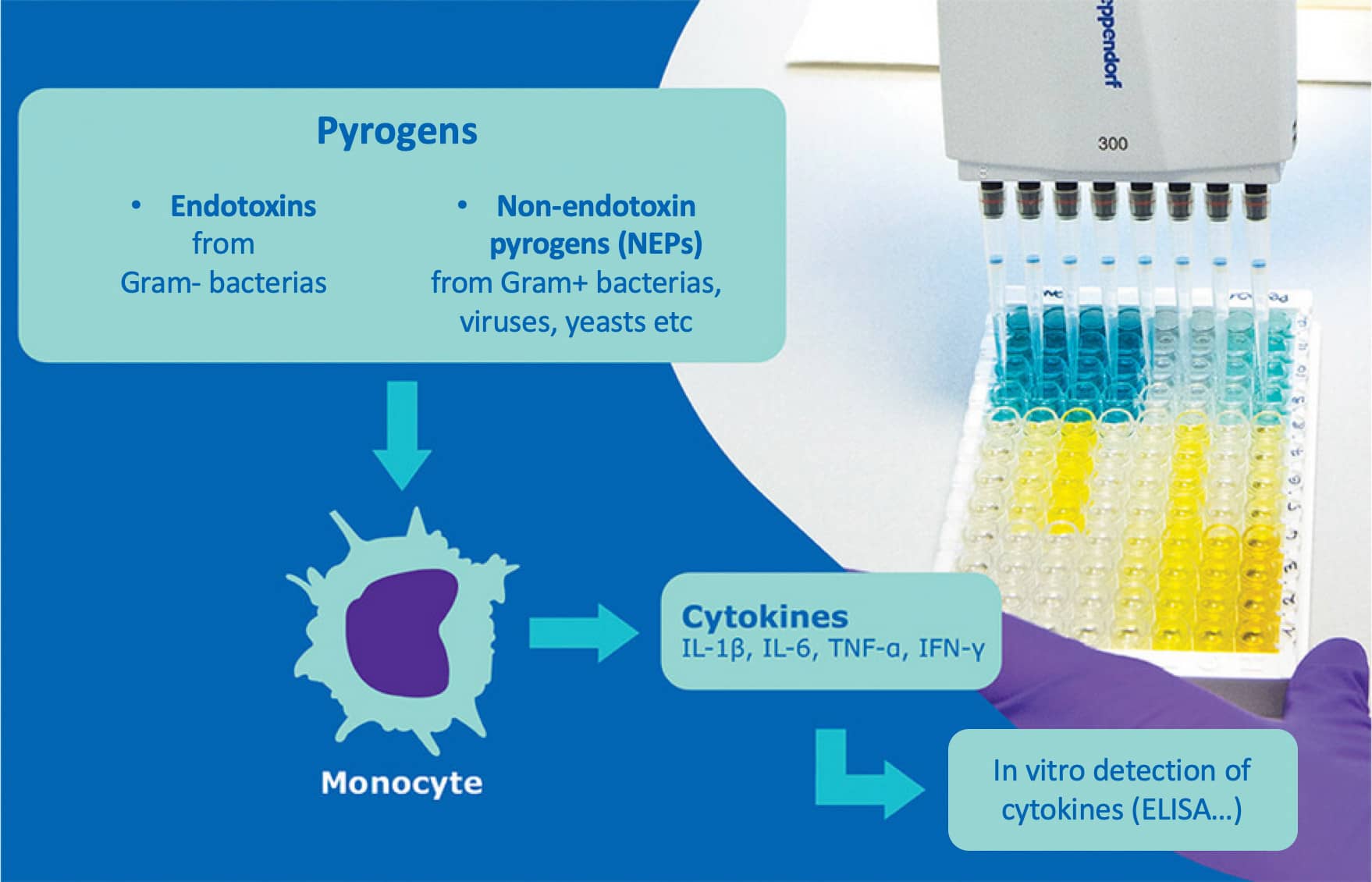
- Another in vitro test, the monocyte activation test (MAT), developed in the 1990s and accepted since 2010 in the European Union, allows the detection of endotoxins but also other pyrogenic contaminants.
This method uses human monocytic cells which, when in contact with pyrogens, produce the inflammatory cytokines (IL1b, TNFa, IL6 etc) responsible for fever, which can be detected in vitro, for example with an ELISA test. Beyond its impact in terms of animal replacement, the MAT is also more suitable for new generations of vaccines that specifically target certain aspects of the human immune system - and are therefore likely to induce fever independently of contamination.
A long overdue replacement
Despite the existence of these alternatives, and of the 2013 European Directive, with its explicit goal to replace methods that have an animal-free alternative (in vitro, in silico...), the abandonment of RPT is still pending, and progress is slow. Despite some success stories, such as Austria, which no longer practices RPT since 2019, after being the biggest European user of rabbit pyrogen tests in 2015, or the total decrease of 49% of rabbit use for this purpose in the EU since 2015, progress remains insufficient: about 30,000 rabbits are still used each year in Europe for pyrogenic tests. More than a third of these tests take place in France, which is now the European country that uses the most rabbits for pyrogenic tests.
The reasons invoked in the EU member states vary, and several measures can and must be taken at different levels to accelerate and facilitate this transition :
|
|
|
|
|
|
Accessibility |
|
|
|
|
|
Technical difficulties |
|
|
The cost of alternative methods |
|
|
|
A real effort of will seems to be needed on the part of the pharmaceutical industry to be informed and trained in alternative methods to RPT, and to implement them in their structures in a way that is adapted to their needs.
It also seems essential that the abandonment of RPT be a general effort, led by the competent authorities on an international scale. Indeed, differences in regulatory protocols between countries still result in numerous tests on rabbits and unnecessary repetitions. For example, the European Pharmacopoeia, which lists the tests (in vivo and in vitro) for safety and efficacy of "medicines", is currently being revised so that the RPT will no longer appear among the methods referenced in its 12th version, which will be published in 2025. For this, 60 texts will be modified.
In the same spirit, the different actors present at the conference (China, Japan, India, Brazil, USA, etc.) have expressed their willingness to abandon the RPT if the alternatives are validated. It therefore appears more necessary than ever to accumulate scientific evidence of the usefulness of in vitro pyrogen testing for the broadest possible applications.
