On 31 January 2024, La Fondation Droit Animal, Éthique et Sciences (LFDA) honoured Dr Alexandra Benchoua with the Alfred Kastler Biology Prize 2023, which encourages the use of non-animal methods in research. She was rewarded for her work on induced pluripotent stem cells (iPSCs), which enable the replacement of animals in pre-clinical experiments. The FC3R had the privilege of talking to the prize winner to learn more about her project and the prospects it has to offer.
Alexandra Benchoua is a research Director at the Institute for Stem cell Therapy and Exploration of Monogenic diseases (I-STEM) at Genopole. Her laboratory uses human pluripotent stem cells to model rare genetic diseases, including neurodevelopmental disorders of the autistic spectrum (Lesch-Nyhan disease, Phelan-McDermid syndrome). She is also responsible for the Stem-CARE platform, which consists of 6 technology platforms offering services such as bioproduction, screening and high-throughput sequencing.

From rodent to human stem cells, her career in neuroscience
After completing her thesis on strokes using murine models at Henri Mondor University Hospital, Dr Benchoua joined the CEA in Orsay for a post-doc on Huntington's disease. While developing primary cultures of rat neurons, Dr Benchoua encountered challenges of repeatability and reproducibility due to the heterogeneity of the cell sources. These difficulties were decisive in Dr Benchoua's choice to favour non-animal methods.
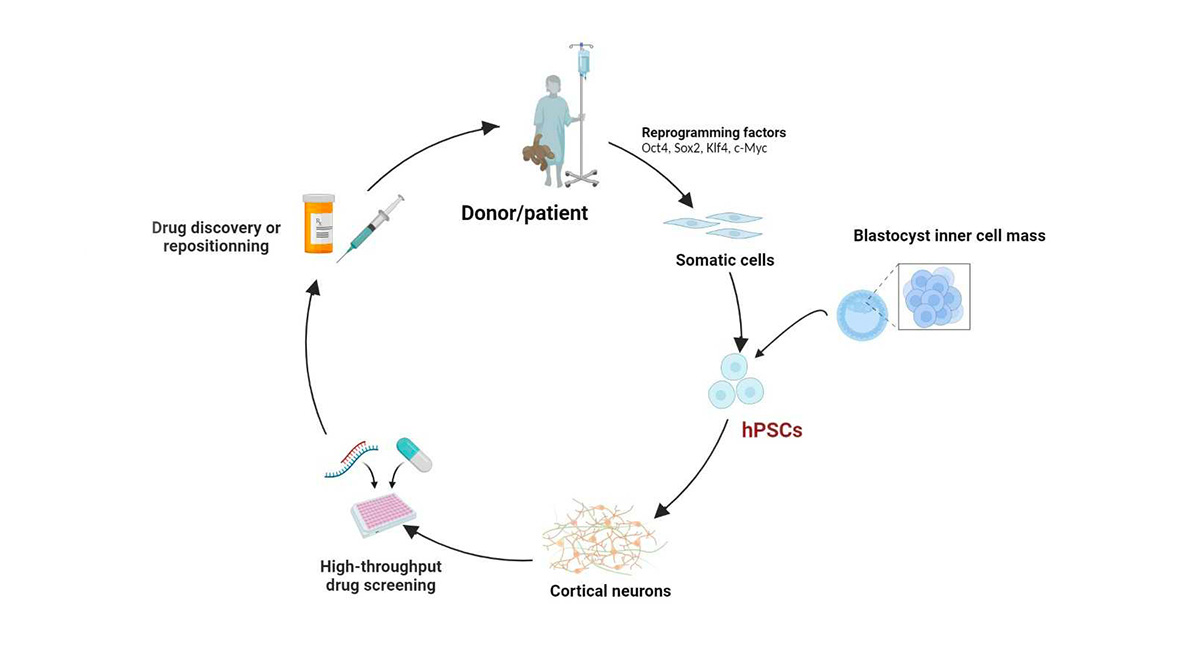
Thus, in 2005, she moved to Edinburgh to work at the forefront of human pluripotent embryonic stem cells (ESCs) development, to focus on studying the signals that guide the development of ESCs towards neuronal differentiation. In 2006, Dr Yamanaka (2012 Nobel Prize in Physiology or Medicine) revolutionized the field of pluripotent stem cells by discovering the method for reprogramming adult cells using transcription factors (Takahashi et al., 2006; Takahashi et al., 2007). This allowed researchers to work with human pluripotent stem cells without relying on embryos. Returning to France, Dr Benchoua set up her research team at the I-STEM, and started to work with this method.
From blood sampling to therapeutic molecule screening
Since then, she and her team have been developing neurons from induced pluripotent stem cells (iPSCs) derived from patients, in order to conduct high-throughput screening of molecules with potential therapeutic activity. This is the project that lead her to be awarded by the LFDA prize.
Through close cooperation with clinicians and patient associations, the laboratory is able to work in direct contact with patients presenting various pathologies. Cells sampling from patients is achieved through a simple blood draw, from which the white blood cells (PBMCs) are subsequently isolated.
Using Dr Yamanaka's method, the laboratory reprograms these cells into pluripotent stem cells (iPSCs), which are then differentiated into neurons using successive mediums containing morphogens. By manipulating these factors, it is possible to obtain different neuronal identities (such as prefrontal cortex neurons) relevant to the studied pathology.
The production of neural progenitors requires two months, after which they can be frozen to form large cell banks for future use. Differentiation into neurons takes two weeks, and 4 to 6 more weeks are necessary to obtain a functionally mature neuronal network.
The neurons are then used by the laboratory for high-throughput screening to evaluate the effect of a large number of molecules. Alexandra Benchoua and her team work with repositioning molecules banks (molecules that are or have been on the market, or abandoned during phase 3 of clinical trials) and with industries willing to screen their own chemical libraries to identify new potential drugs.
To assess the efficacy of a candidate molecule, the team carries out transcriptomic analyses, measures neuronal excitability and uses high-throughput imaging (neuronal branching, soma size, localization of proteins of interest, etc.).
While studying Phelan-McDermid syndrome, a rare genetic disorder caused by a SHANK3 loss of function, Dr Benchoua observed that lithium could induce an increase in SHANK3 expression and its relocation to synpases. This discovery led to the launch of a clinical trial, which is currently in phase 3 under the direction of Professor Richard Delorme.
A more ethical and relevant method for human cellular biology
This method of replacing animals with cells directly derived from patients has several advantages according to Dr. Benchoua:
-
High-throughput screening of a chemical library requires a very large number of neurons, which can be quickly and easily obtained in large quantities from iPSCs. Using animal cells would require an extremely large number of rodent embryos, that would have to be at the same developmental stage, and then dissected to isolate neural tissue, resulting in an immense cost in terms of animal lives, time, resources, and finances.
-
By working directly with patient cells, the possibility of cell therapy can be envisaged, which is not possible with animal cells. Given the strict regulations in this field, it is also necessary to minimize as much as possible the use of xenogenic compounds (which can lead to contamination by endotoxins, bacteria, mycoplasma, etc.) in culture mediums. This means that serum is not used - and is replaced by synthetic mediums composed of small molecules and recombinant human proteins, like recombinant EGF (epidermal growth factor) - even if animal products such as albumin or matrix, produced from mouse cells, are still being used.
-
Human iPSCs, with their human genome, epigenome and transcriptome, enable the modeling of neurons with characteristics that are specific to humans (e.g. fragility) or that are absent or limited in rodents (e.g. neurons of the prefrontal cortex).
-
The use of cells derived directly from patients makes it possible to develop personalized medicine that takes into account the patient’s unique characteristics, which is impossible with cells derived from congenic rodents. Screening can then be used to adapt treatments according to response.
Models increasingly representative of target tissues
The cultures developed by the laboratory for high-throughput screening are relatively simple (2D) and consist of a single type of neurons. While they serve as an excellent model for this application, they lack the complexity needed to understand the influence of other cells and the environment on neurons.
Therefore, Alexandra Benchoua and her team are working on the development of more complex systems to validate drug candidates identified during screening. These organoids provide a more representative 3D model of neuronal architecture. Further levels of complexity can be considered, such as the development of assembloids to bring together neurons from different brain regions, or the co-culture on chips of organoids from different organs.
Making these models more representative, and therefore more relevant to human health, could concurrently reduce reliance on animals for preclinical studies. In this regard, it would also be interesting to explore the possibility of cell culture without animal products.