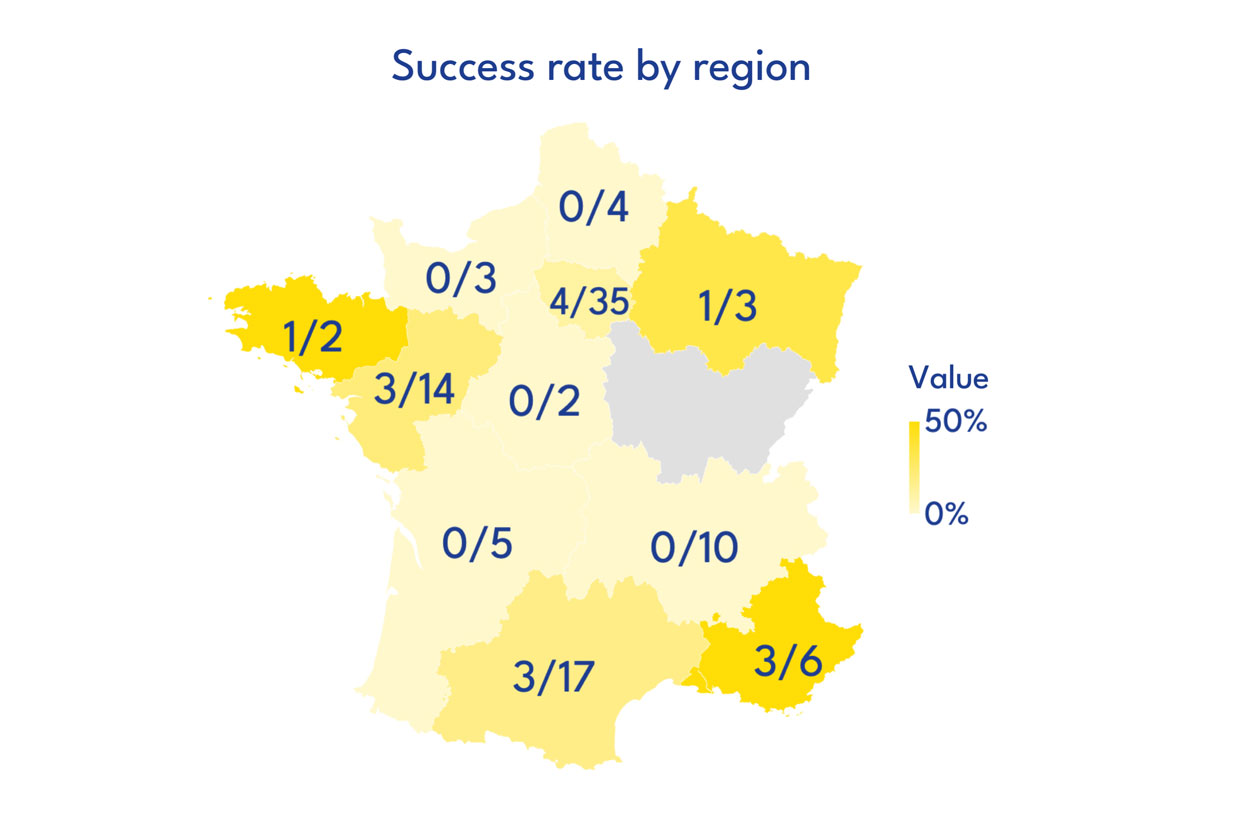
RESEARCH TOPICS FUNDED
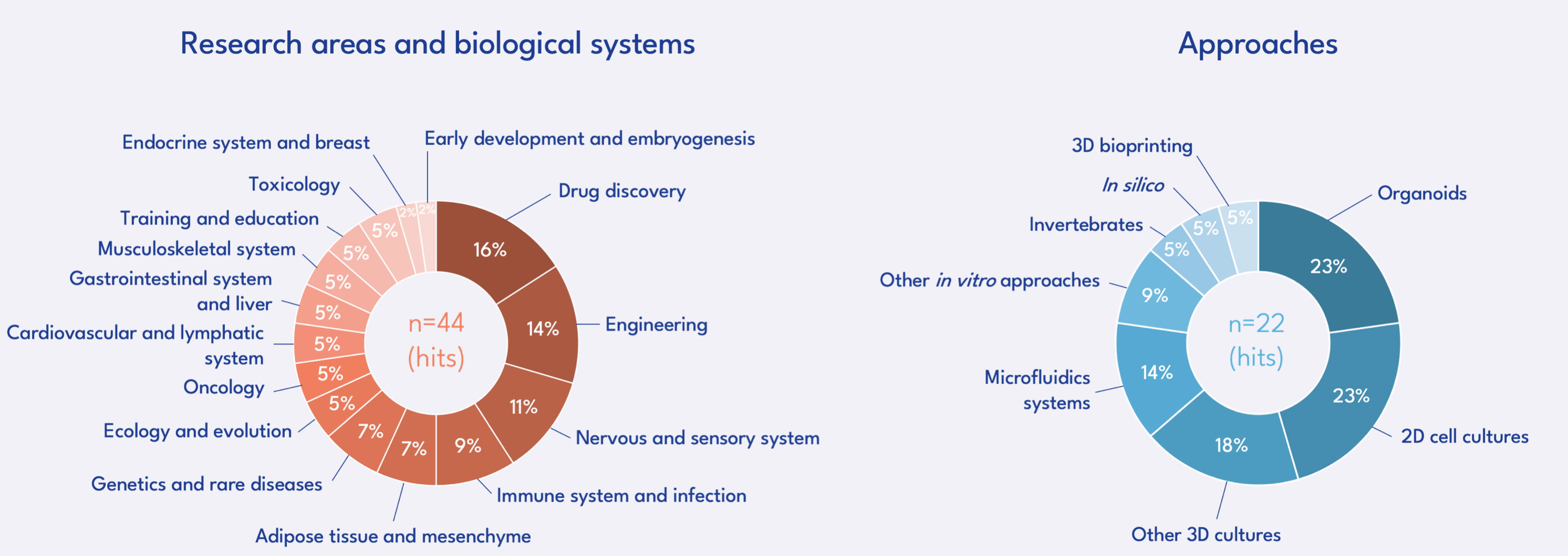
Call for projects of the FC3R – Results publication in April 2025
With this call for projects, the FC3R wanted to promote innovative and cooperative projects, proposing a new strategy, method or technology favouring the Replacement - total, relative or partial - of animals used for scientific purposes: development of alternative in vitro, in silico or in chemico methods, or replacement of animal-derived products (fetal calf serum, extracts of basal membranes, antibodies), but also development of training programs, optimisation of existing methods, or implementation of tools to facilitate sharing (resources, protocols, experimental data) and transfer of knowledge to enhance the adoption and dissemination of these approaches within the scientific community.
101 projects were submitted to this call for proposals. The Scientific Committee selected 15 projects for a total funding of €699 500 by the FC3R.

3D-DISC : Development of a 3D model of the intervertebral disc for the evaluation of regenerative therapies.
Abstract:
Low back pain, the leading cause of disability worldwide, is mainly linked to degeneration of the intervertebral discs. Current treatments, such as painkillers and surgical approaches, do not treat the degenerative biological processes. In contrast, regenerative medicine aims to slow down these processes and restore a healthy disc. In order to reduce the use of animal models for the evaluation of these new therapies, the 3D-DISC project will develop an intervertebral disc model using biofabrication technology. In this in vitro model, we will compare the ability of cells and extracellular vesicles to slow down the degenerative process.
The project is carried out by : Catherine LE VISAGE.
Funding allocated:
The project 3D-DISC is allocated a funding of € 48 500.
3D-MICROTECH : Producing Heterogenous Microenvironments in 3D Cell Culture : Technical and Training Approaches.
Abstract:
Two-thirds of anti-cancer drug candidates that work in mice fail in human trials. Moreover, resistance to validated treatments remains a major challenge in patients. The solution to these problems is largely linked to the possibility of reproducing the heterogeneity of the original cancer, with non-identical tumor zones that favor tumor aggressiveness. Our innovative project focuses on (1) the development of human cell culture models that reproduce tumor heterogeneity by acting on the environment in which cancer cells live, and (2) theoretical and practical training in the production of tumors in the laboratory that conform to reality. This project will be promoted in the scientific and clinical arenas while respecting the animal kingdom.
The project is carried out by : Sophie LELIèVRE.
Funding allocated:
The project 3D-MICROTECH is allocated a funding of € 48 500.
A-CELLHERD : Establishment of a herd of porcine pluripotent stem cells to replace animal use for genetic research and genomic selection.
Abstract:
Current global changes are forcing us to rethink our livestock systems to reduce pressure on ecosystems, increase food and health security and better take animal welfare into consideration. To do this, we use genetic, nutritional or environmental levers that allow us to better adapt farm animals to these new objectives. But to operate these levers effectively, it is necessary to be able to predict their effects on animals. For this and in order to avoid resorting to invasive sampling and animal experimentation, we propose to replace animals with herds of pluripotent cell lines. These cells make it possible to produce various tissues and mini-organs in vitro and in particular facilitate the study of health or nutrition traits in relation to genetic, nutritional and environmental determinants.
The project is carried out by : Hervé ACLOQUE.
Funding allocated:
The project A-CELLHERD is allocated a funding of € 39 000.
Adiponet : Human adipose Tissue Organoids: Development and Standardization of Freezing/Thawing and Culture Methods for Biobanks.
Abstract:
Metabolic diseases and chronic inflammatory bowel diseases have conquered the world in recent decades, with the westernization of lifestyle and eating habits. A common factor in these pathologies would be the role played by adipose tissue in the control of metabolism and inflammation in these pathologies. Research uses thousands of mice each year to elucidate this role, but it is known that the adipose tissue of these animals is different from that of humans. The AdipoNet project aims to create a procedure for freezing/thawing human adipose tissue allowing its transport and exchanges between laboratories, but also the culture of adipose tissue from tissue samples of patients that have been thawed, and the establishment of biobanks for medical research from these cultures.
The project is carried out by : Anne BOULOUMIE.
Funding allocated:
The project Adiponet is allocated a funding of € 48 500.
CARDIOCELL : Patient-specific pacemaker-like iPSC-derived cardiomyocytes (hiPSC-PMs): a new cellular model for sino-atrial node dysfunction treatment.
Abstract:
The human-iPSC technology has provided researchers with a unique tool to derive disease-specific stem cells for disease modeling purposes . The rationale is to derive hiPSCs from patients and then differentiate them in vitro into affected cell types, thereby recapitulating the disease in a Petri dish without using animal model. Disease modelling has enabled the study of a range of human diseases in the lab. The main goal of this approach is to use these unique ‘‘Petri dish’’ disease models, harboring the patient genome and cellular context to identify new therapeutic approaches to treat symptomatic sinus node dysfunction (SND), the most common bradyarrhythmia and the leading cause for over 500,000 electric pacemaker devices implanted yearly in Europe and U.S
The project is carried out by : Pietro MESIRCA.
Funding allocated:
The project CARDIOCELL is allocated a funding of € 48 500.
CellMorphTox : Cell Morphology for chemical toxicity assessment.
Abstract:
Thanks to innovative fluorescence-printed cell imaging techniques, such as ‘Cell Painting’, it is now possible to analyse the morphological changes in cells induced by exposure to a chemical molecule, in a systematic process that paves the way for screening campaigns for chemical compound libraries. In this context, the CellMorphTox project aims to collect this type of data and develop a computational method for identifying a cell morphology ‘signature’ for a set of compounds sharing the same phenotypic profile that is harmful to human health. This tool will be accessible to the scientific community via an online platform and will help to reduce animal testing, in line with the 3Rs principles (Replacement, Reduction, Refinement). It will be able to guide the authorities in assessing future chemical risks.
The project is carried out by : Olivier TABOUREAU.
Funding allocated:
The project CellMorphTox is allocated a funding of € 48 500.
FELINT : New cellular models to better understand the specific interactions between T. gondii and its definitive feline host.
Abstract:
Toxoplasmosis is a zoonotic disease caused by the parasite Toxoplasma gondii (Tg), which has serious consequences for the immunocompromised and the fetus in the case of primary infection in pregnant women. Tg can infect all warm-blooded animals, but its sexual reproduction takes place only in cats, resulting in the fecal excretion of infectious parasites into the environment, inducing animal and human contaminations. It is therefore essential to develop effective prophylactic or therapeutic strategies against Tg in cats to block the parasite excretion and thus prevent environmental contaminations to improve public health. Our research project proposes to develop and use new cell culture approaches, as an alternative to using animals, to increase our knowledge of the life cycle of Tg in cats to potentially identify new prevention strategies.
The project is carried out by : Delphine LE ROUX.
Funding allocated:
The project FELINT is allocated a funding of € 35 000.
GlioTumoroids : In vitro modelling of glioblastoma physiopathology in human cortico-endothelial organoids.
Abstract:
Glioblastoma (GB) is an aggressive brain tumour with a poor prognosis, and its interactions with the surrounding cellular environment remain poorly understood. Traditionally, GB studies rely on mouse models, but this approach is costly, time-consuming, and raises ethical concerns. This project proposes an innovative in vitro alternative: an organoid system combining human cortical and endothelial cells (CO+EO) that replicates the vascularized brain tissue. Using this model, we will explore GB cell interactions with different tissue types and investigate the role of the CELF2 gene in tumour aggressiveness. This research aims to decipher the molecular mechanisms that influence GB migration and growth, paving the way for new treatments while reducing the use of animal models.
The project is carried out by : Michele BERTACCHI.
Funding allocated:
The project GlioTumoroids is allocated a funding of € 48 500.
Habemuscl : Advanced human-based preclinical platform for neuromuscular disease modeling.
Abstract:
Neuromuscular diseases (NMDs) like muscular dystrophies lack effective preclinical models to study their underlying mechanisms and test treatments. While animal models have been used for decades, they often fail to accurately reflect human conditions, leading to high treatment failures in clinical trials. Human cell models are a promising alternative, but they often don’t mature enough to fully replicate disease processes. This project aims to create an advanced, human-based platform to model skeletal muscle tissue under both healthy and pathological conditions. By combining 2D and 3D cell cultures with electrical and mechanical stimulation, we plan to develop an all-in-one device for analysis of engineered muscle models. These models will be exploited for NMDs modeling, drug testing and personalized medicine, reducing the need for animal models and saving time and research costs.
The project is carried out by : Stefano TESTA.
Funding allocated:
The project Habemuscl is allocated a funding of € 45 000.
HOBIT : Hepatic Organoids and their Biological Intrinsic Timing.
Abstract:
Circadian (about-a-day) rhythms are central in health and diseases and originates from the molecular circadian clock (CC) present in all body cells. The liver CC is key for metabolic health as it is perturbed in liver diseases. There is a need for alternative to animal experiment to improve pre-clinical results transferability to Human. In this framework, synchronized Human induced pluripotent stem cells (hiPSCs) derived in 3D-liver-organoids is a good experimental alternative but nothing is known about its temporal properties. Using a protocol free of animal derivatives, we aim at precisely characterizing the temporal/circadian characteristics of synchronized hiPSCs-derived 3D-liver-organoids. This work will (1) make 3D-liver-organoids more physiologically relevant, (2) reduce the number of animals used in pharmacological research and (3) will be a great asset to improve precision and personalised medicine.
The project is carried out by : Daniel MAUVOISIN.
Funding allocated:
The project HOBIT is allocated a funding of € 48 500.
ICOPRIM : Improving Cerebellar Organoid Protocols for Reliable and Innovative Disease Modeling.
Abstract:
The cerebellum, known for motor coordination, also plays critical roles in memory, language, and emotion. Congenital cerebellar malformations are associated with serious neurodevelopmental disorders, which are the focus of our research. Despite advances in genetics, many cases remain unexplained, and no therapies are available. To address this, we are developing in vitro models grown from stem cells, called organoids, which mimic early human cerebellar development. By enhancing precision, reproducibility and tractability, this project aims to establish these models as a robust alternative to animal models, to better understand disease mechanisms and move forward to therapeutic strategies.
The project is carried out by : Marion COOLEN.
Funding allocated:
The project ICOPRIM is allocated a funding of € 48 500.
MINIBRAIN : Modeling human neurodegeneration on a chip for ethical and successful drug development.
Abstract:
Neurodegenerative diseases are reaching epidemic levels in industrialized countries, affecting over 7 million people in Europe. This number is set to double every 20 years as the population is aging. Despite this, no effective therapies exist. Drug development faces major challenges as traditional animal testing have low predictive value for human efficacy, needlessly sacrificing countless animals. Recent advances in human stem cell technology combined with organ-on-chip microsystems are offering a new hope. These systems can accurately replicate human organs at the microscale, enhancing the physiological relevance of today’s in vitro models, while providing ethical alternatives to animal experimentation. Our MINIBRAIN project aims to develop this next-generation of humanized brain-on-chip devices, paving the way for more effective and ethical drug development practices.
The project is carried out by : Maxime CAZORLA.
Funding allocated:
The project MINIBRAIN is allocated a funding of € 47 000.
OBANI : Obtaining antibodies for immunohistochemistry and western blotting without animals.
Abstract:
Phage Display: An Innovative Method for Developing Specific Antibodies Phage display is a modern technology that enables the rapid production of antibodies targeting denatured proteins, such as those analyzed in immunohistochemistry or western blot. It uses bacteriophages (viruses) modified to display antibodies on their surface, allowing direct and precise selection against the linear epitopes of denatured proteins. This method is fast, avoids the use of animals, and offers great flexibility to adapt to specific experimental conditions. The antibodies produced are highly specific, effective, and can be optimized to meet the needs of research or diagnostics. Phage display is therefore an ethical and powerful solution for developing reliable tools tailored to detecting proteins in denaturing conditions.
The project is carried out by : Pierre MARTINEAU.
Funding allocated:
The project OBANI is allocated a funding of € 48 500.
Optimello : Optimize the use of the insect Galleria mellonella as alternative infection model for bacterial pathogens.
Abstract:
The Optimello project explores the potential of Galleria mellonella (Gm) as an alternative to vertebrate animals in bacterial infection research. Adhering to the 3Rs principles (Replace, Reduce, Refine), this easy-to-maintain model is cost-effective, ethical, and scalable. Gm possesses an innate immune system similar to that of humans. Optimello has three main objectives: to produce a fully annotated Gm genome, to map host-pathogen interactions through transcriptomic studies, and to create an online platform for data sharing and protocol standardization. By overcoming technical challenges, the project aims to establish Gm as a reference model for research, while minimizing the use of vertebrate animals.
The project is carried out by : Yoann AUGAGNEUR.
Funding allocated:
The project Optimello is allocated a funding of € 48 500.
ReMiRa : Development of cell substitutes suitable for routine use in rabies-related activities.
Abstract:
Rabies, an always-fatal viral infection, remains a major public health problem worldwide, with around 59,000 human deaths annually following transmission from infected animals. Accurate laboratory diagnosis is therefore essential to confirm the disease in animals, assess its distribution, and manage potentially exposed individuals. Currently, samples for inter-laboratory comparisons, as well as positive laboratory controls, are obtained from experimentally infected mice. Given the considerable progress made in the production and use of 3D cell-based systems, we propose to develop adapted and specific laboratory models to reduce and replace the use of mice for rabies virus production, collection of infected material, viral isolation and pathophysiology studies.
The project is carried out by : Marine WASNIEWSKI.
Funding allocated:
The project ReMiRa is allocated a funding of € 48 500.
3D-DISC : Development of a 3D model of the intervertebral disc for the evaluation of regenerative therapies.
3D-MICROTECH : Producing Heterogenous Microenvironments in 3D Cell Culture : Technical and Training Approaches.
A-CELLHERD : Establishment of a herd of porcine pluripotent stem cells to replace animal use for genetic research and genomic selection.
Abstract:
Low back pain, the leading cause of disability worldwide, is mainly linked to degeneration of the intervertebral discs. Current treatments, such as painkillers and surgical approaches, do not treat the degenerative biological processes. In contrast, regenerative medicine aims to slow down these processes and restore a healthy disc. In order to reduce the use of animal models for the evaluation of these new therapies, the 3D-DISC project will develop an intervertebral disc model using biofabrication technology. In this in vitro model, we will compare the ability of cells and extracellular vesicles to slow down the degenerative process.
The project is carried out by : Catherine LE VISAGE.
Funding allocated:
The project 3D-DISC is allocated a funding of € 48 500.
Abstract:
Two-thirds of anti-cancer drug candidates that work in mice fail in human trials. Moreover, resistance to validated treatments remains a major challenge in patients. The solution to these problems is largely linked to the possibility of reproducing the heterogeneity of the original cancer, with non-identical tumor zones that favor tumor aggressiveness. Our innovative project focuses on (1) the development of human cell culture models that reproduce tumor heterogeneity by acting on the environment in which cancer cells live, and (2) theoretical and practical training in the production of tumors in the laboratory that conform to reality. This project will be promoted in the scientific and clinical arenas while respecting the animal kingdom.
The project is carried out by : Sophie LELIèVRE.
Funding allocated:
The project 3D-MICROTECH is allocated a funding of € 48 500.
Abstract:
Current global changes are forcing us to rethink our livestock systems to reduce pressure on ecosystems, increase food and health security and better take animal welfare into consideration. To do this, we use genetic, nutritional or environmental levers that allow us to better adapt farm animals to these new objectives. But to operate these levers effectively, it is necessary to be able to predict their effects on animals. For this and in order to avoid resorting to invasive sampling and animal experimentation, we propose to replace animals with herds of pluripotent cell lines. These cells make it possible to produce various tissues and mini-organs in vitro and in particular facilitate the study of health or nutrition traits in relation to genetic, nutritional and environmental determinants.
The project is carried out by : Hervé ACLOQUE.
Funding allocated:
The project A-CELLHERD is allocated a funding of € 39 000.
Adiponet : Human adipose Tissue Organoids: Development and Standardization of Freezing/Thawing and Culture Methods for Biobanks.
CARDIOCELL : Patient-specific pacemaker-like iPSC-derived cardiomyocytes (hiPSC-PMs): a new cellular model for sino-atrial node dysfunction treatment.
CellMorphTox : Cell Morphology for chemical toxicity assessment.
Abstract:
Metabolic diseases and chronic inflammatory bowel diseases have conquered the world in recent decades, with the westernization of lifestyle and eating habits. A common factor in these pathologies would be the role played by adipose tissue in the control of metabolism and inflammation in these pathologies. Research uses thousands of mice each year to elucidate this role, but it is known that the adipose tissue of these animals is different from that of humans. The AdipoNet project aims to create a procedure for freezing/thawing human adipose tissue allowing its transport and exchanges between laboratories, but also the culture of adipose tissue from tissue samples of patients that have been thawed, and the establishment of biobanks for medical research from these cultures.
The project is carried out by : Anne BOULOUMIE.
Funding allocated:
The project Adiponet is allocated a funding of € 48 500.
Abstract:
The human-iPSC technology has provided researchers with a unique tool to derive disease-specific stem cells for disease modeling purposes . The rationale is to derive hiPSCs from patients and then differentiate them in vitro into affected cell types, thereby recapitulating the disease in a Petri dish without using animal model. Disease modelling has enabled the study of a range of human diseases in the lab. The main goal of this approach is to use these unique ‘‘Petri dish’’ disease models, harboring the patient genome and cellular context to identify new therapeutic approaches to treat symptomatic sinus node dysfunction (SND), the most common bradyarrhythmia and the leading cause for over 500,000 electric pacemaker devices implanted yearly in Europe and U.S
The project is carried out by : Pietro MESIRCA.
Funding allocated:
The project CARDIOCELL is allocated a funding of € 48 500.
Abstract:
Thanks to innovative fluorescence-printed cell imaging techniques, such as ‘Cell Painting’, it is now possible to analyse the morphological changes in cells induced by exposure to a chemical molecule, in a systematic process that paves the way for screening campaigns for chemical compound libraries. In this context, the CellMorphTox project aims to collect this type of data and develop a computational method for identifying a cell morphology ‘signature’ for a set of compounds sharing the same phenotypic profile that is harmful to human health. This tool will be accessible to the scientific community via an online platform and will help to reduce animal testing, in line with the 3Rs principles (Replacement, Reduction, Refinement). It will be able to guide the authorities in assessing future chemical risks.
The project is carried out by : Olivier TABOUREAU.
Funding allocated:
The project CellMorphTox is allocated a funding of € 48 500.
FELINT : New cellular models to better understand the specific interactions between T. gondii and its definitive feline host.
GlioTumoroids : In vitro modelling of glioblastoma physiopathology in human cortico-endothelial organoids.
Habemuscl : Advanced human-based preclinical platform for neuromuscular disease modeling.
Abstract:
Toxoplasmosis is a zoonotic disease caused by the parasite Toxoplasma gondii (Tg), which has serious consequences for the immunocompromised and the fetus in the case of primary infection in pregnant women. Tg can infect all warm-blooded animals, but its sexual reproduction takes place only in cats, resulting in the fecal excretion of infectious parasites into the environment, inducing animal and human contaminations. It is therefore essential to develop effective prophylactic or therapeutic strategies against Tg in cats to block the parasite excretion and thus prevent environmental contaminations to improve public health. Our research project proposes to develop and use new cell culture approaches, as an alternative to using animals, to increase our knowledge of the life cycle of Tg in cats to potentially identify new prevention strategies.
The project is carried out by : Delphine LE ROUX.
Funding allocated:
The project FELINT is allocated a funding of € 35 000.
Abstract:
Glioblastoma (GB) is an aggressive brain tumour with a poor prognosis, and its interactions with the surrounding cellular environment remain poorly understood. Traditionally, GB studies rely on mouse models, but this approach is costly, time-consuming, and raises ethical concerns. This project proposes an innovative in vitro alternative: an organoid system combining human cortical and endothelial cells (CO+EO) that replicates the vascularized brain tissue. Using this model, we will explore GB cell interactions with different tissue types and investigate the role of the CELF2 gene in tumour aggressiveness. This research aims to decipher the molecular mechanisms that influence GB migration and growth, paving the way for new treatments while reducing the use of animal models.
The project is carried out by : Michele BERTACCHI.
Funding allocated:
The project GlioTumoroids is allocated a funding of € 48 500.
Abstract:
Neuromuscular diseases (NMDs) like muscular dystrophies lack effective preclinical models to study their underlying mechanisms and test treatments. While animal models have been used for decades, they often fail to accurately reflect human conditions, leading to high treatment failures in clinical trials. Human cell models are a promising alternative, but they often don’t mature enough to fully replicate disease processes. This project aims to create an advanced, human-based platform to model skeletal muscle tissue under both healthy and pathological conditions. By combining 2D and 3D cell cultures with electrical and mechanical stimulation, we plan to develop an all-in-one device for analysis of engineered muscle models. These models will be exploited for NMDs modeling, drug testing and personalized medicine, reducing the need for animal models and saving time and research costs.
The project is carried out by : Stefano TESTA.
Funding allocated:
The project Habemuscl is allocated a funding of € 45 000.
HOBIT : Hepatic Organoids and their Biological Intrinsic Timing.
ICOPRIM : Improving Cerebellar Organoid Protocols for Reliable and Innovative Disease Modeling.
MINIBRAIN : Modeling human neurodegeneration on a chip for ethical and successful drug development.
Abstract:
Circadian (about-a-day) rhythms are central in health and diseases and originates from the molecular circadian clock (CC) present in all body cells. The liver CC is key for metabolic health as it is perturbed in liver diseases. There is a need for alternative to animal experiment to improve pre-clinical results transferability to Human. In this framework, synchronized Human induced pluripotent stem cells (hiPSCs) derived in 3D-liver-organoids is a good experimental alternative but nothing is known about its temporal properties. Using a protocol free of animal derivatives, we aim at precisely characterizing the temporal/circadian characteristics of synchronized hiPSCs-derived 3D-liver-organoids. This work will (1) make 3D-liver-organoids more physiologically relevant, (2) reduce the number of animals used in pharmacological research and (3) will be a great asset to improve precision and personalised medicine.
The project is carried out by : Daniel MAUVOISIN.
Funding allocated:
The project HOBIT is allocated a funding of € 48 500.
Abstract:
The cerebellum, known for motor coordination, also plays critical roles in memory, language, and emotion. Congenital cerebellar malformations are associated with serious neurodevelopmental disorders, which are the focus of our research. Despite advances in genetics, many cases remain unexplained, and no therapies are available. To address this, we are developing in vitro models grown from stem cells, called organoids, which mimic early human cerebellar development. By enhancing precision, reproducibility and tractability, this project aims to establish these models as a robust alternative to animal models, to better understand disease mechanisms and move forward to therapeutic strategies.
The project is carried out by : Marion COOLEN.
Funding allocated:
The project ICOPRIM is allocated a funding of € 48 500.
Abstract:
Neurodegenerative diseases are reaching epidemic levels in industrialized countries, affecting over 7 million people in Europe. This number is set to double every 20 years as the population is aging. Despite this, no effective therapies exist. Drug development faces major challenges as traditional animal testing have low predictive value for human efficacy, needlessly sacrificing countless animals. Recent advances in human stem cell technology combined with organ-on-chip microsystems are offering a new hope. These systems can accurately replicate human organs at the microscale, enhancing the physiological relevance of today’s in vitro models, while providing ethical alternatives to animal experimentation. Our MINIBRAIN project aims to develop this next-generation of humanized brain-on-chip devices, paving the way for more effective and ethical drug development practices.
The project is carried out by : Maxime CAZORLA.
Funding allocated:
The project MINIBRAIN is allocated a funding of € 47 000.
OBANI : Obtaining antibodies for immunohistochemistry and western blotting without animals.
Optimello : Optimize the use of the insect Galleria mellonella as alternative infection model for bacterial pathogens.
ReMiRa : Development of cell substitutes suitable for routine use in rabies-related activities.
Abstract:
Phage Display: An Innovative Method for Developing Specific Antibodies Phage display is a modern technology that enables the rapid production of antibodies targeting denatured proteins, such as those analyzed in immunohistochemistry or western blot. It uses bacteriophages (viruses) modified to display antibodies on their surface, allowing direct and precise selection against the linear epitopes of denatured proteins. This method is fast, avoids the use of animals, and offers great flexibility to adapt to specific experimental conditions. The antibodies produced are highly specific, effective, and can be optimized to meet the needs of research or diagnostics. Phage display is therefore an ethical and powerful solution for developing reliable tools tailored to detecting proteins in denaturing conditions.
The project is carried out by : Pierre MARTINEAU.
Funding allocated:
The project OBANI is allocated a funding of € 48 500.
Abstract:
The Optimello project explores the potential of Galleria mellonella (Gm) as an alternative to vertebrate animals in bacterial infection research. Adhering to the 3Rs principles (Replace, Reduce, Refine), this easy-to-maintain model is cost-effective, ethical, and scalable. Gm possesses an innate immune system similar to that of humans. Optimello has three main objectives: to produce a fully annotated Gm genome, to map host-pathogen interactions through transcriptomic studies, and to create an online platform for data sharing and protocol standardization. By overcoming technical challenges, the project aims to establish Gm as a reference model for research, while minimizing the use of vertebrate animals.
The project is carried out by : Yoann AUGAGNEUR.
Funding allocated:
The project Optimello is allocated a funding of € 48 500.
Abstract:
Rabies, an always-fatal viral infection, remains a major public health problem worldwide, with around 59,000 human deaths annually following transmission from infected animals. Accurate laboratory diagnosis is therefore essential to confirm the disease in animals, assess its distribution, and manage potentially exposed individuals. Currently, samples for inter-laboratory comparisons, as well as positive laboratory controls, are obtained from experimentally infected mice. Given the considerable progress made in the production and use of 3D cell-based systems, we propose to develop adapted and specific laboratory models to reduce and replace the use of mice for rabies virus production, collection of infected material, viral isolation and pathophysiology studies.
The project is carried out by : Marine WASNIEWSKI.
Funding allocated:
The project ReMiRa is allocated a funding of € 48 500.